Abstract

Background Acute myeloid leukemia (AML) has an aggressive course and a historically dismal prognosis. For many patients, hematopoietic stem cell transplantation (HSCT) represents the best option for cure, but access, utilization and health inequities on a global scale remain poorly elucidated.
Methods Estimates of AML incident cases in 2016 were obtained from the Global Burden of Disease (GBD) 2019 study. HSCT activities were collected from 2009-2016 by the Worldwide Network for Blood and Marrow Transplantation (WBMT) through the European Society for Blood and Marrow Transplantation (EBMT), the Center for International Blood and Marrow Transplantation (CIBMTR), the Asian Pacific Blood and Marrow Transplant Group (APBMT), the Australasian Bone Marrow Transplant Recipient Registry (ABMTRR), the Eastern Mediterranean Blood and Marrow Transplant Group (EMBMT), the Latin American Bone Marrow Transplantation Group (LABMT), the African Blood and Marrow Transplantation Group (AFBMT) and the Cell Therapy Transplant Canada (CTTC). The primary endpoint was to analyze the global and regional use and utilization of HSCT (number of HSCT/ number of incident cases) for AML. Secondary outcomes included trend from 2009 to 2016, donor type, stem cell source and remission status.
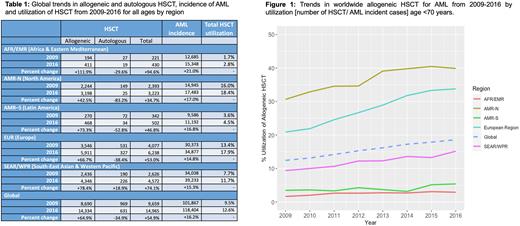
Results Global AML incidence has steadily increased, from 101,867 (95% uncertainty interval (UI): 90,240-107,950) in 2009 to 118,404 (95% UI 103,620-125,950) in 2016 (+16.2%; see Table 1). Over the same period globally, a 54.9% increase from 9,659 to 14,965 HSCT/year was observed, driven by an increase in allogeneic (+64.9%) with a simultaneous reduction in autologous (-34.9%) HSCT. While highest numbers of HSCT continue to be performed in high- income regions, the largest increases were seen in resource-constrained regions [+94.6% in Africa/East Mediterranean Region (AFR/EMR)] as compared to high-income regions [+34.7% in America-Nord Region (AMR-N)]. HSCT utilization was skewed towards high-income regions [in 2016: AMR-N 18.4%, Europe (EUR) 17.9%, South East Asia/Western Pacific Region (SEAR/WPR) 11.7%, America-South Region (AMR-S) 4.5% and AFR/EMR 2.8%]. For patients <70 years of age this difference in utilization was widened (Figure 1); AMR-N has the highest allogeneic utilization rate, increasing from 2009 to 2016 (30.6% to 39.9%, respectively) with continued low utilization observed in AFR/EMR (1.7% to 2.9%) and AMR-S (3.5% to 5.4%, respectively). In all regions, total HSCT for AML in 1st complete remission (CR1) increased (from 44.1% to 59.0%). Patterns of donor stem cell source from related versus unrelated donors varied widely by geographic region. SEAR/WPR has had a +130.2% increase in related donor from 2009 to 2016 and >95% HSCT donors in AFR/EMR are from related as compared to a predilection for unrelated HSCT in AMR-N and EUR. Globally, stem cell source for allogeneic HSCT was predominantly from peripheral blood with increasing use from 2009 to 2016. Autologous HSCT decreased in all regions except in SEAR/WPR (+18.9%, see Table 1)
Conclusions HSCT remains a central curative treatment modality in AML and an understanding of global access, practices and unmet needs is important. Allogeneic HSCT for AML is rising globally but there are marked variations in regional utilization and practices, including types of graft source. Resource-constrained regions have the largest growth in HSCT use, but utilization rates remain low with a predilection for familial related donor sources and are typically offered in CR1. Further studies are necessary to elucidate the reasons, including economic factors, to understand and address these health inequalities and improve discrepancies in use of HSCT as a potentially curative treatment globally.
Disclosures
Cowan:Abbvie: Consultancy, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy; BMS: Consultancy, Research Funding; EUSA: Consultancy; GSK: Consultancy; Harpoon: Research Funding; Janssen: Consultancy, Research Funding; Nektar: Research Funding; Sanofi: Research Funding; Secura Bio: Consultancy. Greinix:Gilead, Novartis, Sanofi, Cellgene: Consultancy; Amgen, Gilead, Novartis, Sanofi, Takeda, Therakos: Speakers Bureau. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Mohty:Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Takeda: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Adaptive Biotechnologies: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria. Ciceri:Kite Pharma: Consultancy. Gomez-De Leon:AbbVie: Honoraria, Other: advisory board. Hamad:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Percival:Pfizer: Research Funding; Trillium: Research Funding; Oscotec: Research Funding; Cardiff Oncology: Research Funding; Glycomimetics: Research Funding; Biosight: Research Funding; Celgene/BMS: Research Funding; Abbvie: Research Funding; Ascentage: Research Funding; Telios: Research Funding. Srivastava:Roche: Research Funding; Pfizer: Research Funding; Sanofi: Other: The study was supported by Sanofi, Research Funding; NovoNordisk: Research Funding. Szer:Novartis: Consultancy, Honoraria, Speakers Bureau; Biocryst: Consultancy, Honoraria, Speakers Bureau; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL: Consultancy, Honoraria, Speakers Bureau; Eli-Lilly: Consultancy, Honoraria, Speakers Bureau; Sobi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau. Wood:Pfizer: Other: Research funding to my institution; Genentech: Other: Research funding to my institution. Snowden:Mallinckrodt: Speakers Bureau; Novartis: Speakers Bureau; Gilead: Speakers Bureau; Janssen and Jazz: Speakers Bureau; Medac: Membership on an entity's Board of Directors or advisory committees; Kiadis: Other: clinical trial IDMC membership . Weisdorf:FATE Therapeutics: Other: Research Support; Incyte: Other: Research support. Pasquini:Kite: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. Sureda:Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Jannsen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; GenMab: Consultancy, Honoraria; Pierre Fabre: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria. Atsuta:Novartis Pharma KK: Honoraria; Kyowa Kirin Co., Ltd: Honoraria; AbbVie GK: Honoraria; Astellas Pharma Inc.: Honoraria; Mochida Pharmaceutical Co., Ltd.: Honoraria; Meiji Seika Pharma Co, Ltd.: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal